I am the most interested in The question number 16. I am the most interested in this because I am a swimmer and i really love the ocean and all the sea animals that live in it so I am fascinated by whats at the bottom of the ocean. A hypothesis for this would be, If many more live so low under the water then there must be more animals at the ocean bottom.
1. What is the galaxy?
2. What is the difference between animals and plants?
3. How was Earth made?
4. What made plants?
5.What are humans?
6. What is matter?
7. What is lava?
8. What are earthquakes?
9. How are tsunamis formed?
10. Why are tsunamis formed in hot climates?
11. What is at the bottom of the Earth?
12. What is sweat?
13. Is there ghosts?
14. What grow bamboos?
15. Could humans ever fly?
16. When can animals talk?
17. What is leaves?
18. What makes stone?
19. How many stars are there?
20. How many galaxies are there?
Tuesday, September 29, 2015
Monday, September 28, 2015
Identifying Questions and Hypotheses
Identifying Questions and Hypotheses
The experiment that i choose to write about was when Robert Hooke used a microscope to observe cells. He was requested to create a series of microscopical studies from King Charles the 2nd of England. He studied even more than he had to, like he looked at fabrics, leaves, and glass. And he was the first person to see cells in things like corks. The question was whether all things had living cells, like corks. The hypothesis was, If humans have living cells then plants and insects must also have cells.
http://www.science-of-aging.com/timelines/hooke-history-cell-discovery.php
Monday, September 21, 2015
Unit 2 reflection
This unit was about the essentials of biology. It showed that atoms have a nucleus made of protons and neutrons, and electrons surround the nucleus. Protons are positively charged, neutrons have no charge and electrons have a negative charge. The basic of chemical reactions and bonding. What is needed to create a chemical bonding and what is made from it. It also shows what changes in a chemical bonding. Showed the pH of things. And that a pH below 7 is acidic and a pH above 7 is a base. Things that are acidic include, lemon juice, milk, and tomato juice. Some things that are a base is laundry detergent, and some cleaning supplies. And lastly it thought us about the 4 macro-molecules. These include carbohydrates, proteins, nucleic acids and lipids. They all have very different jobs, but all of them have important roles in the human body. And how enzymes help with many reactions.
Some strengths that i thought I had about this unit was the structure of the atoms. The pH scale, and the 4 macro-molecules. I thought i could learn more about these and thought they were more interesting. Chemical bonding was one I didn't understand as much as the other. I think I didn't understand this one as well because there was a lot information to connect to the main idea. But i think working in groups on labs and projects helped me understand some of these topics more than i would by myself.
I learned a lot from this unit and labs. But some specifics were how the 4 macro-molecules really work together to help build the body. And also some benefits they have, and how much of each one you should consume. I also learned how to work in groups to get the results you are looking for, like in a lab.
I think i am a better student today than yesterday because i learned a lot more that i can take and apply to real life situations.
I want to learn more about the process of chemical bonding. Like all the things needed to do one and what are made. I also want to learn about enzymes.

Some strengths that i thought I had about this unit was the structure of the atoms. The pH scale, and the 4 macro-molecules. I thought i could learn more about these and thought they were more interesting. Chemical bonding was one I didn't understand as much as the other. I think I didn't understand this one as well because there was a lot information to connect to the main idea. But i think working in groups on labs and projects helped me understand some of these topics more than i would by myself.
I learned a lot from this unit and labs. But some specifics were how the 4 macro-molecules really work together to help build the body. And also some benefits they have, and how much of each one you should consume. I also learned how to work in groups to get the results you are looking for, like in a lab.
I think i am a better student today than yesterday because i learned a lot more that i can take and apply to real life situations.
I want to learn more about the process of chemical bonding. Like all the things needed to do one and what are made. I also want to learn about enzymes.



Saturday, September 19, 2015
Cheese Lab
Time to Curdle
| ||||
Curdling agent
|
Chymosin
|
Rennin
|
Buttermilk
|
Milk (control)
|
Acid
|
5
|
5
|
20
| |
Base
|
15
|
10
| ||
Cold
| ||||
Hot
|
20
|
33
| ||
Average of Controls
|
13
|
15
|
Conclusion
In this lab we asked the question, what are the optimal conditions and curdling agents for making cheese. From this lab we found that a curdling agent of acid and added rennin and chymosin would be the best for making cheese. This was the best because it got a time of only 5 minutes while the base got 10 and 15, and hot got 20 and 33. The added buttermilk only worked for the acid, but the time was And this is a quantitative type of observation.This data does and does not support our claim because we said that added chymosin which is a rennin enzyme would curdle the milk the best and it did. But we also said that the temperature for making it curdle would be hot but acid worked the best.
Our data was unexpected because almost all of the other groups curdling agents got a time but ours got n/a. Due to this we might have had a error with the amount of buttermilk we added. This might have happened because it was really hard to measure a small amount of something that was really thick. So we might not have had the right amount of buttermilk in our test tubes. This probably affected that it didn't curdle cause we had too little. Another error that we had was the temperature of the hot test tube. The test tube that was supposed to be hot, but it didn't become as hot as we expected. This happened because the hot water bath was room temperature and not hot. It affected the milk so it didn't have enough heat to curdle. Due to these errors, in future experiments I would recommend that we would have had a better way to measure the buttermilk, and spend more time to get the amount correct. And for the second error, instead of leaving the test tube in the hot water we could have put the test tube in someone's hand or between arm and body.
This lab was done to demonstrate what agents are the best to curdle milk. From this lab I learned that Acid with added rennin and chymosin was the best to curdle milk, and how the enzymes help curdle the milk which helps me understand the concept of making cheese, and how enzymes and bacteria are important in the making of many foods. Based on my experience from this lab i could learn how bacteria works and also enzymes and how a small difference between temperature or measurement can change something dramatically.
Tuesday, September 15, 2015
Sweetness lab
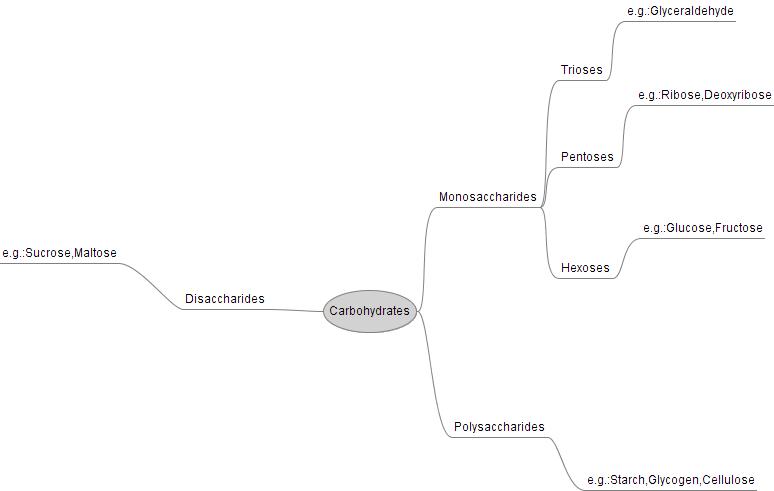
The purpose of this lab was to demonstrate how the the structure of a carbohydrate affects its taste. We thought that the disacchrides would have the highest degree of sweetness. Monosaccharides taste sweet and have one ring, disacchrides also taste sweet but have to rings, and polysacchrides have three rings and taste starchy and plain. For monosacchrides we had lactose and fructose and their degree of sweetness for lactose was 50 and fructose was 90. The dissacharides in this experiment was sucrose and glucose and the degree of sweetness we observed was 100 for sucrose and 95 for glucose. We had many polysacchrides but for one of them, maltose had a degree of sweetness of 25 and for all of them the range was between 0-75. This is evidence that our claim was correct because the dissachrides had 95 and 100, while the others had a range of 0-95.
The molecular structure of carbohydrates like glucose allows for the creation of long strings of monosaccharides into polysaccharides which the cell can use to store excess sugar. In plants they can be used to give cellulose fibers their strength.
All tasters gave different ratings for the different samples. The ratings could be different because everyone has different taste buds. Another reason is that one person might have started with a sweet on and the sweetness might have affected the next sample, while another person might have started with a starchy one and that would have made the next sample taste a little starchy instead of sweet like the first person. Also all people have a different rating on how they think sweet is, like some person might think glucose is 95 and another person might think its 85.
The thing that makes us taste sweetness is when we eat or drink something sweet, it simulates proteins in our sweet-responding cells. And then they send a signal to the brain. A reason that people could rank sweetness differently, is that some people might have a slow signal sent from the taste buds to the brain or not as strong or many taste buds.

Carbohydrate
|
Type of
Carbohydrate
|
Degree of
Sweetness
|
Color
|
Texture
|
Other observations
|
Sucrose
|
Disaccharide
|
100
|
White
|
Granular
|
Taste and looks like sugar
|
Glucose
|
Disaccharide
|
95
|
White
|
Granular
|
Grainy
|
Fructose
|
Monosaccharide
|
90
|
White
|
Grainy
|
Really sweet looks like sugar
|
Galactose
|
Polysaccharide
|
75
|
White
|
Powder
|
Looks like powder sugar
|
Maltose
|
Polysaccharide
|
25
|
Brown
|
Chunky
|
Hard chunks
|
Lactose
|
Monosaccharide
|
50
|
White
|
Har, powdery
|
Hard, compressed powder
|
Starch
|
Polysaccharide
|
0
|
White
|
Powder
|
Powder the sticky, thick
|
Cellulose
|
Polysaccharide
|
5
|
WHite
|
Powder
|
Powdery looks like powder sugar
|
Friday, September 4, 2015
Subscribe to:
Comments (Atom)
